Saggin' Spuds (Osmosis)

Osmosis is a tricky one for kids to understand, mostly because they get confused about the concentration of water versus the concentration of a substance that's dissolved in the water.
Saggin' Spuds
It's Osmosis in Action!
Osmosis is a tricky one for kids to understand, mostly because they get confused about the concentration of water versus the concentration of a substance that's dissolved in the water.
 |
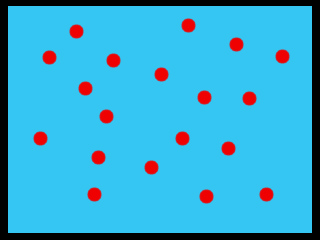 |
These two containers are the same size. One is full of water. The one is full of water with salt in it.
Questions
Q: Which one has more water?
A: The one on the left (solid blue)! In the container on the right, there is space being taken up by salt that could be taken up by water.
Q: So, which one has the higher concentration of water?
A: The one with more water in it! That's the one on the left.
Q: If the space between the containers above was a semi-permeable membrane (permeable to water), which way would water go by osmosis?
A: From Left to Right, as shown here:
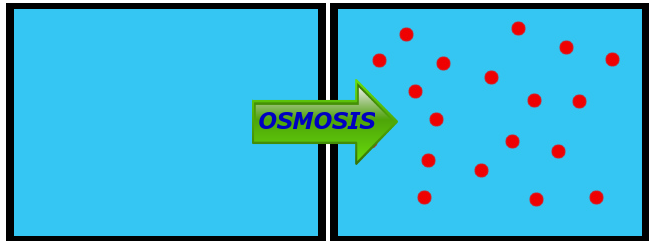
Water is going to move from where there is more water (higher concentration) to where there is less water (lower concentration), same as with any diffusing substance. The thing that makes osmosis tricky for a lot of kids is that they get focused on the dissolved substance, not the water itself. Osmosis IS diffusion - it's just the diffusion specifically of WATER.
Materials List
- 1 Tablespoon of Salt
- 1 Cup of Water
- A Bowl
- A Spoon
- Potato Slices (CAUTION: Get permission/help from an adult before cutting potato slices) about 1/4 inch thick.
Procedure
1. Pour the water into the bowl and add the salt. Stir until the salt dissolves.
2. Obtain 2 slices of potato. Carefully hold them with your fingertips and gently bend them back and forth to get a feel for how rigid they are. Don't break them!
3. Place the potatoes into the salt water and wait 15 minutes.
4. When time is up, pick up the potato slices like before and gently bend them again. Do they feel different?
What's Going On?
You probably noticed that when you took the potato slices out of the salt water and bent them again, that they were much less rigid than before - way more "bendy"! Well, that potato is made of thousands of tiny cells, which are like little bags filled (mostly) with water. When you put the potato into salt water, water moved OUT of the potato's cells and into the water in the bowl. As the water left the cells, they became more saggy and limp. This movement of water is called osmosis!
Extensions
Can you think of a way to make the potato slice rigid again? How about trying this experiment with something besides a potato slice?
© Hema and Eric Bulmer. All rights reserved.
